Zeroth Law
If system A is at thermal
equilibrium with system B, and
B is at thermal equilibrium with
system C, then
A is at thermal equilibrium with
C. This is more a matter of logic
than of physics. Two systems are at thermal equilibrium if they have the same
temperature. If A and
B have the same temperature, and
B and
C have the same temperature, then
A and C have the same
temperature.
Video Lesson - Zeroth Law of Thermodynamics
The significant consequence of the Zeroth Law is that, when a hotter object and
a colder object are placed in contact with one another, heat will flow from the
hotter object to the colder object until they are in thermal equilibrium.
First Law
Consider an isolated system—that is, one where heat and energy neither enter nor
leave the system. Such a system is doing no work, but we associate with it a
certain internal energy, U,
which is related to the kinetic energy of the molecules in the system, and
therefore to the system’s temperature. Internal energy is similar to potential
energy in that it is a property of a system that is doing no work, but has the
potential to do work.
Video Lesson - First Law of Thermodynamics
The First Law tells us that the internal energy of a system increases if heat is
added to the system or if work is done on the system and decreases if the system
gives off heat or does work. We can express this law as an equation:
?U = ?Q + ?W
where U signifies internal energy,
Q signifies heat, and
W signifies work.
The First Law is just another way of stating the law of conservation of energy.
Both heat and work are forms of energy, so any heat or work that goes into or
out of a system must affect the internal energy of that system.
Example
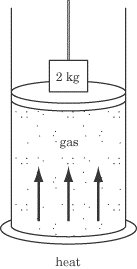
Some heat is added to a gas container that is topped by a movable piston. The
piston is weighed down with a 2 kg mass. The piston rises a distance of 0.2 m at
a constant velocity. Throughout this process, the temperature of the gas in the
container remains constant. How much heat was added to the container?
The key to answering this question is to note that the temperature of the
container remains constant. That means that the internal energy of the system
remains constant (?U = 0),
which means that, according to the First Law,
?Q + ?W = 0.
By pushing the piston upward, the system does a certain amount of work,
?W,
and this work must be equal to the amount of heat added to the system,
?Q.
The amount of work done by the system on the piston is the product of the force
exerted on the piston and the distance the piston is moved. Since the piston
moves at a constant velocity, we know that the net force acting on the piston is
zero, and so the force the expanding gas exerts to push the piston upward must
be equal and opposite to the force of gravity pushing the piston downward. If
the piston is weighed down by a two-kilogram mass, we know that the force of
gravity is:
F = (mg)(2kg)(9.8 m/s2) = 19.6 N
Since the gas exerts a force that is equal and opposite to the force of gravity,
we know that it exerts a force of 19.6 N
upward. The piston travels a distance of 0.2
m, so the total work done on the piston is:
W = Fd = (19.6N)(0.2m) = 3.92J
Since
?W
in the equation for the First Law of Thermodynamics is positive when work is
done on the system and negative when work is done by the system, the value of
?W
is –3.92 J. Because
?U = 0?,
we can conclude that
Q = 3.92
J, so 3.92 J
of heat must have been added to the system to make the piston rise as it did.
Second Law
There are a number of equivalent forms of the Second Law, each of which sounds
quite different from the others. Questions about the Second Law on
Physics will invariably be qualitative. They will usually ask that you identify
a certain formulation of the Second Law as an expression of the Second Law.
Video Lesson - Second Law of Thermodynamics
The Second Law in Terms of Heat Flow
Perhaps the most intuitive formulation of the Second Law is that heat flows
spontaneously from a hotter object to a colder one, but not in the opposite
direction. If you leave a hot dinner on a table at room temperature, it will
slowly cool down, and if you leave a bowl of ice cream on a table at room
temperature, it will warm up and melt. You may have noticed that hot dinners do
not spontaneously get hotter and ice cream does not spontaneously get colder
when we leave them out.
The Second Law in Terms of Heat Engines
One consequence of this law, which we will explore a bit more in the section on
heat engines, is that no machine can work at 100% efficiency: all
machines generate some heat, and some of that heat is always lost to the
machine’s surroundings.
The Second Law in Terms of Entropy
The Second Law is most famous for its formulation in terms of entropy.
The word entropy was coined in the 19th century as a technical term for
talking about disorder. The same principle that tells us that heat spontaneously
flows from hot to cold but not in the opposite direction also tells us that, in
general, ordered systems are liable to fall into disorder, but disordered
systems are not liable to order themselves spontaneously.
Imagine pouring a tablespoon of salt and then a tablespoon of pepper into a jar.
At first, there will be two separate heaps: one of salt and one of pepper. But
if you shake up the mixture, the grains of salt and pepper will mix together. No
amount of shaking will then help you separate the mixture of grains back into
two distinct heaps. The two separate heaps of salt and pepper constitute a more
ordered system than the mixture of the two.
Next, suppose you drop the jar on the floor. The glass will break and the grains
of salt and pepper will scatter across the floor. You can wait patiently, but
you’ll find that, while the glass could shatter and the grains could scatter, no
action as simple as dropping a jar will get the glass to fuse back together
again or the salt and pepper to gather themselves up. Your system of salt and
pepper in the jar is more ordered than the system of shattered glass and
scattered condiments.
Entropy and Time
You may have noticed that Newton’s Laws and the laws of kinematics are
time-invariant. That is, if you were to play a videotape of kinematic motion in
reverse, it would still obey the laws of kinematics. Videotape a ball flying up
in the air and watch it drop. Then play the tape backward: it goes up in the air
and drops in just the same way.
Video Lesson - What is Entropy
By contrast, you’ll notice that the Second Law is not time-invariant: it tells
us that, over time, the universe tends toward greater disorder. Physicists
suggest that the Second Law is what gives time a direction. If all we had were
Newton’s Laws, then there would be no difference between time going forward and
time going backward. So we were a bit inaccurate when we said that entropy
increases over time. We would be more accurate to say that time moves in the
direction of entropy increase.
Third Law
It is impossible to cool a substance to absolute zero. This law is irrelevant as
far as Physics is concerned, but we have included it for the sake of
completeness.
Next to display next topic in the chapter.
Practice Questions
Video Lessons and 10 Fully Explained Grand Tests
Large number of solved practice MCQ with explanations. Video Lessons and 10 Fully explained Grand/Full Tests.