Now let’s look more closely at pi bonds. As we mentioned earlier in this chapter,
pi (p) bonds result from the sideways overlap of p orbitals, and pi
orbitals are defined by the region above and below an imaginary line connecting
the nuclei of the two atoms. Keep in mind that pi bonds never occur unless a
sigma bond has formed first, and they may form only if unhybridized p
orbitals remain on the bonded atoms. Also, they occur when sp or sp2
hybridization is present on central atom but not sp3
hybridization.
Below, we show the formation of a set of sp2 orbitals. This
molecule would contain a double bond, like ethene. Notice again how the first
set of figures form the sp2 atomic orbital, the hybrid, and
the last figure shows full hybridization:
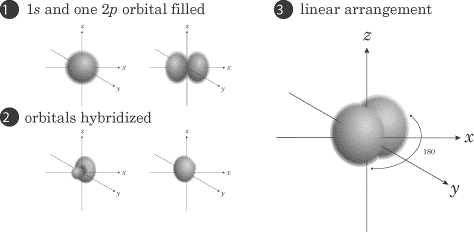
The set of p orbitals that are unhybridized are not shown in this
depiction:
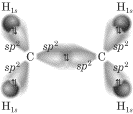
A different view, which doesn’t show the hydrogens and centers on the C atoms,
shows the unhybridized p orbitals that create the sideways overlap that’s
necessary to create the double pi bond:
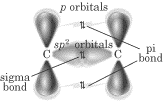
Here’s how it looks with all the pieces put together:
