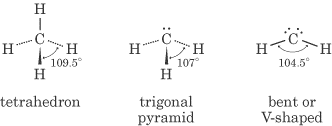
As you can see from the table, atoms that have normal valence—meaning atoms that
have no more than four structural electron pairs and obey the octet rule (and
have no lone pairs)—are tetrahedral. For instance, look at methane, which is CH4:
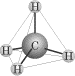
Ammonia (NH3), which has three sigma bonds and a lone pair, however,
is trigonal pyramidal:
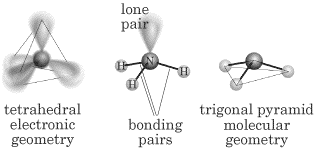
Water (H2O) has two lone pairs and its molecular geometry is “bent,”
which is also called V shaped:

So as you can see, lone pairs have more repulsive force than do shared electron pairs, and thus they force the shared pairs to squeeze more closely together.
As a final note, you may remember that we mentioned before that only elements
with a principal energy level of 3 or higher can expand their valence and
violate the octet rule. This is because d electrons are necessary to make
possible bonding to a fifth or sixth atom. In XeF4, there are two
lone pairs and four shared pairs surrounding Xe, and two possible arrangements
exist:
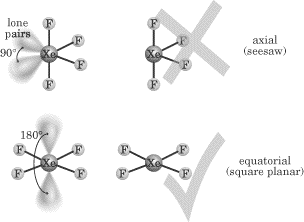
In the axial arrangement, shared pairs are situated “top and bottom.” In the
equatorial arrangement, shared pairs surround Xe. The equatorial arrangement is
more stable since the lone pairs are 180° apart and this minimizes their
repulsion. In both molecular arrangements, the electronic geometry is
octahedral, with 90° angles. The top figure has a molecular geometry known as
“seesaw,” while the bottom figure has a molecular geometry that is more stable,
known as square planar.