Lesson: Chapter - 19
The Discovery of the Atom
The idea that matter is made up of infinitely small, absolutely simple,
indivisible pieces is hardly new. The Greek thinkers Leucippus and Democritus
suggested the idea a good 100 years before Aristotle declared it was nonsense.
However, the idea has only carried scientific weight for the past 200 years, and
it only really took off in the past century.
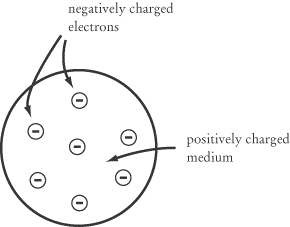
Thompson’s “Plum Pudding” Model
The first major discovery that set off modern atomic theory was that atoms
aren’t in fact the smallest things that exist. J. J. Thompson discovered the
electron in 1897, which led him to posit a “plum pudding” model
(a.k.a. the “raisin pudding” model) for the atom. Electrons are small negative
charges, and Thompson suggested that these negative charges are distributed
about a positively charged medium like plums in a plum pudding. The negatively
charged electrons would balance out the positively charged medium so that each
atom would be of neutral charge.
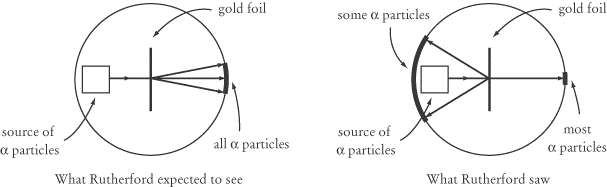
Rutherford’s Gold Foil Experiment
In a series of experiments from 1909 to 1911, Ernest Rutherford established that
atoms have nuclei. His discovery came by accident and as a total surprise. His
experiment consisted of firing alpha particles, which we will examine in
more detail shortly, at a very thin sheet of gold foil. Alpha particles consist
of two protons and two neutrons: they are relatively massive
(about 8000 times as massive as an electron), positively charged particles. The
idea of the experiment was to measure how much the alpha particles were
deflected from their original course when they passed through the gold foil.
Because alpha particles are positively charged and electrons are negatively
charged, the electrons were expected to alter slightly the trajectory of the
alpha particles. The experiment would be like rolling a basketball across a
court full of marbles: when the basketball hits a marble, it might deflect a bit
to the side, but, because it is much bigger than the marbles, its overall
trajectory will not be affected very much. Rutherford expected the deflection to
be relatively small, but sufficient to indicate how electrons are distributed
throughout the “plum pudding” atom.
To Rutherford’s surprise, most of the alpha particles were hardly deflected at
all: they passed through the gold foil as if it were just empty space. Even more
surprising was that a small number of the alpha particles were deflected at
180°, right back in the direction they came
from.
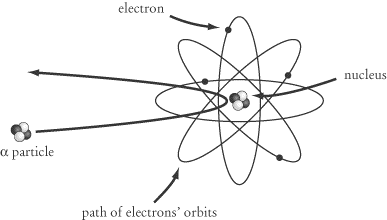
This unexpected result shows that the mass of an atom is not as evenly
distributed as Thompson and others had formerly assumed. Rutherford’s
conclusion, known as the Rutherford nuclear model, was that the mass of
an atom is mostly concentrated in a nucleus made up of tightly bonded
protons and neutrons, which are then orbited by electrons. The electromagnetic
force pulls the electrons into orbit around the nucleus in just the way that the
gravitational force pulls planets into orbit around the sun.
The radius of an atom’s nucleus is about 1/10,000 the radius of the atom itself.
As a result, most of the alpha particles in Rutherford’s gold foil experiment
passed right through the sheet of gold foil without making contact with
anything. A small number, however, bumped into the nucleus of one of the gold
atoms and bounced right back.
Back
Next
Next to display next topic in the chapter.
Practice Questions
Video Lessons and 10 Fully Explained Grand Tests
Large number of solved practice MCQ with explanations. Video Lessons and 10 Fully explained Grand/Full Tests.