Lesson: Chapter - 13
Electric Forces, Fields, and Potential
Democritus, a Greek philosopher of the 5th
century B.C., was the first to propose that all things are made of indivisible
particles called
atoms. His hypothesis was only half right.
Video Lesson -
The things we call atoms today are in fact made up of three different kinds of particles:
protons,
neutrons, and
electrons. Electrons are much smaller
than the other two particles. Under the influence of the electronic force,
electrons orbit the
nucleus of the atom, which contains protons and
neutrons.
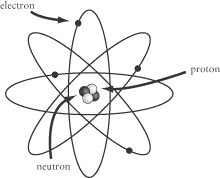
Protons and electrons both carry electric charge, which causes them to be
attracted to one another.
In most atoms, there are as many electrons as there
are protons, and the opposite charges of these two kinds of particle balance
out. However, it is possible to break electrons free from their orbits about the
nucleus, causing an imbalance in charge. The movement of free electrons is the
source of everything that we associate with electricity, a phenomenon whose
power we have learned to harness over the past few hundred years to
revolutionary effect.
Next to display next topic in the chapter.
Practice Questions
Video Lessons and 10 Fully Explained Grand Tests
Large number of solved practice MCQ with explanations. Video Lessons and 10 Fully explained Grand/Full Tests.