Atomic Mass Unit
Because objects on the atomic level are so tiny, it can be a bit unwieldy to
talk about their mass in terms of kilograms. Rather, we will often use the
atomic mass unit (amu, or sometimes just u), which is defined as one-twelfth
of the mass of a carbon-12 atom. That means that 1
amu =
1.6605 × 10-27
kg. We can express the mass of the elementary particles either in kilograms or
atomic mass units:
l amu = 1.6605
× -27
|
Particle |
Mass (kg) |
Mass (amu) |
|
Proton |
1.6725
× -27 |
1.0073 |
|
Neutron |
1.6747
× -27 |
1.0086 |
|
Electron |
9.11
× -31 |
5.4863
× -4 |
As you can see, the mass of electrons is pretty much negligible when calculating
the mass of an atom.
div>
Atomic Number, Neutron Number, and Mass Number
You’re probably somewhat familiar with the periodic table and know that there
are over 100 different chemical elements.
An element is defined by the number of protons in the atomic nucleus. For
instance, a nucleus with just one proton is hydrogen, a nucleus with two protons
is helium, and a nucleus with 92 protons is
uranium, the heaviest naturally occurring element. The number of protons in an
atomic nucleus determines the atomic number,
Z. In an electrically neutral atom
of atomic number Z, there will be
Z protons and
Z electrons.
The number of neutrons in an atomic nucleus determines the neutron number,
N. Different nuclei of the same
atomic number—that is, atoms of the same element—may have different numbers of
neutrons. For instance, the nuclei of most carbon atoms have six protons and six
neutrons, but some have six protons and eight neutrons. Atoms of the same
element but with different numbers of neutrons are called isotopes.
As we saw above, electrons weigh very little in comparison to protons and
neutrons, which have almost identical masses. The sum of the atomic number and
the neutron number, Z +
N, gives us an atom’s mass number,
A.
Chemical Notation
The standard form for writing the chemical symbol of an element,
X, is:
zAX
The element’s mass number is written in superscript, and the atomic number is
written in subscript. You can infer the neutron number by subtracting
A – Z. For instance, we would write
the chemical symbol for the two carbon isotopes, called carbon-12 and carbon-14,
as follows:
carbon-12: 612C
carbon-12: 614C
The same sort of system can be used to represent protons, neutrons, and
electrons individually. Because a proton is the same thing as a hydrogen atom
without an electron, we can represent protons by writing:
11H+
where the + sign shows that the hydrogen
ion has a positive charge due to the absence of the electron. Neutrons are
represented by the letter “n” as follows:
01u
Electrons and positrons, which are positively charged electrons, are
represented, respectively, as follows:
electron: -10e
positron: +1oe
The number in subscript gives the charge of the particle—0 in the case of the neutron and
–1 in the case of the electron. The number
in superscript gives the mass. Though electrons have mass, it is so negligible
in comparison to that of protons and neutrons that it is given a mass number of
0.
Some Other Elementary Particles
On the subject test, you will not need to apply your knowledge of any elementary
particles aside from the proton, the neutron, and the electron. However, the
names of some other particles may come up, and you will at least need to know
what they are.
Quarks are the fundamental building blocks of the protons, neutrons, and
mesons. They generally have positive or negative charges in units of one-third
to two-thirds of the charge of the electron. Protons are neutrons
composed of three quarks. Mesons are composed of a quark–antiquark pair.
Radioactive Decay
Some configurations of protons and neutrons are more stable in a nucleus than
others. For instance, the carbon-12 atom is more stable than the carbon-14 atom.
While carbon-12 will remain stable, carbon-14 will spontaneously transform into
a more stable isotope of nitrogen, releasing particles and energy in the
process. Because these transformations take place at a very steady rate,
archaeologists can date carbon-based artifacts by measuring how many of the
carbon-14 atoms have decayed into nitrogen. These transformations are called
radioactive decay, and isotopes and elements like carbon-14 that undergo
such decay are called radioactive. There are three major kinds of
radioactive decay.
Alpha Decay
When an atom undergoes alpha decay, it sheds an alpha particle,
a,
which consists of two protons and two neutrons. Through alpha decay, an atom
transforms into a smaller atom with a lower atomic number. For instance,
uranium-238 undergoes a very slow process of alpha decay, transforming into
thorium:
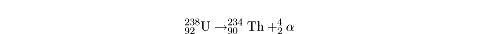
Notice that the combined mass number and atomic number of the two particles on
the right adds up to the mass number and atomic number of the uranium atom on
the left.
Beta Decay
There are actually three different kinds of beta decay—ß-
decay,
ß+
decay, and electron capture—but subject test Physics will only deal with
ß-
decay, the most common form of beta decay. In
ß-
decay, one of the neutrons in the nucleus transforms into a proton, and an
electron and a neutrino,
v,
are ejected. A neutrino is a neutrally charged particle with very little mass.
The ejected electron is called a beta particle,
ß.
The decay of carbon-14 into nitrogen is an example of
ß-
decay:
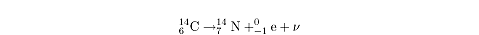
Note that the mass number of the carbon on the left is equal to the sum of the
mass numbers of the nitrogen and the electron on the right:
14 = 14 + 0. Similarly, the atomic number
of the carbon is equal to the sum of the atomic number of the nitrogen and the
electron: 6 = 7 – 1. Because the neutrino
has no charge and negligible mass, its presence has no effect on any aspect of
beta decay that we will study. Still, it’s important that you know the
neutrino’s there.
Gamma Decay
Gamma decay is the most straightforward kind of decay. An element in a
high-energy state can return to a lower energy state by emitting a gamma ray,
?,
which is an electromagnetic photon of very high frequency. No other particles
are ejected and the nucleus doesn’t transform from one element to another. All
we get is an ejected gamma ray, as in this example with technetium:
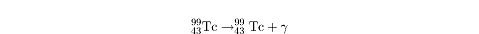
Example
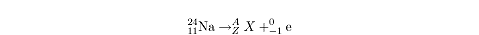
The reaction schematized above is an example of what form of radioactive decay?
What are the values for A, Z, and X?
What form of radioactive decay?
In the above reaction, a sodium nucleus transforms into some other element and
gives off an electron. Electrons are only released in beta decay. A neutrino is
also released but, because its effects are negligible, it is often left out of
the equation.
What are the values for A, Z, and X?
We can calculate A and
Z because the sum of the atomic
numbers and the mass numbers on the right must add up to the atomic number and
the mass number on the left. We can solve for A
and Z with the following equations:
24 = A + 0
11 = Z - 1
So A =
24 and Z =
12. The resulting element is determined by
the atomic number, Z. Consult a
periodic table, and you will find that the element with an atomic number of
12 is magnesium, so
X stands in for the chemical symbol
for magnesium, Mg.
Binding Energy
Atomic nuclei undergo radioactive decay so as to go from a state of high energy
to a state of low energy. Imagine standing on your hands while balancing a box
on your feet. It takes a lot of energy, not to mention balance, to hold yourself
in this position. Just as you may spontaneously decide to let the box drop to
the floor and come out of your handstand, atomic nuclei in high-energy states
may spontaneously rearrange themselves to arrive at more stable low-energy
states.
Nuclear Forces
So far, all the physical interactions we have looked at in this book result from
either the gravitational force or the electromagnetic force. Even the collisions
we studied in the chapters on mechanics are the result of electromagnetic
repulsion between the atoms in the objects that collide with one another.
However, neither of these forces explains why the protons in an atomic nucleus
cling together. In fact, the electromagnetic force should act to make the
protons push away from one another, not cling together. Explaining how things
work on the atomic level requires two additional forces that don’t act beyond
the atomic level: the strong and weak nuclear forces. The strong
nuclear force binds the protons and neutrons together in the nucleus. The weak
nuclear force governs beta decay. You don’t need to know any of the math
associated with these forces, but you should know what they are.
Mass Defect
As we have discussed, the mass of a proton is
1.0073 amu and the mass of a neutron is
1.0086 amu. Curiously, though, the mass of an alpha particle, which
consists of two protons and two neutrons, is not
2(1.0073) + 2(1.0086) = 4.0318 amu, as one might expect, but rather
4.0015 amu. In general, neutrons and
protons that are bound in a nucleus weigh less than the sum of their masses. We
call this difference in mass the mass defect,
?m,
which in the case of the alpha particle is 4.0318
– 4.0015 = 0.0202 amu.
Einstein’s Famous Equation
The reason for this mass defect is given by the most famous equation in the
world:
E = mc2
As we discussed in the section on relativity, this equation shows us that mass
and energy can be converted into one another.
The strong nuclear force binds the nucleus together with a certain amount of
energy. A small amount of the matter pulled into the nucleus of an atom is
converted into a tremendous amount of energy, the binding energy, which
holds the nucleus together. In order to break the hold of the strong nuclear
force, an amount of energy equal to or greater than the binding energy must be
exerted on the nucleus. For instance, the binding energy of the alpha particle
is:
E = mc2 = (3.354 × 10-29kg)(3.0 × 108m/s)2
= 3.0 × 10-12J
Note that you have to convert the mass from atomic mass units to kilograms in
order to get the value in joules. Often we express binding energy in terms of
millions of electronvolts, MeV, per nucleon. In this case,
3.0 × 10-12
J = 18.7 MeV. Because there are four
nucleons in the alpha particle, the binding energy per nucleon is
18.7/4 = 4.7 MeV/nucleon.
Example
A deuteron, a particle consisting of a proton and a neutron, has a binding
energy of 1.12 MeV per nucleon. What is the mass of the deuteron?
Since there are two nucleons in a deuteron, the binding energy for the deuteron
as a whole is
1.12 × 2 = 2.24MeV. That energy, converted into mass, is:
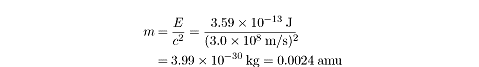
The mass of a free proton plus a free neutron is
1.0073 + 1.0086 = 2.0159 amu. The mass of the deuteron will be
0.0024 amu less than this amount, since
that is the amount of mass converted into energy that binds the proton and the
neutron together. So the deuteron will weigh
2.0159 – 0.0024 = 2.0135 amu.
Decay Rates
On subject test Physics, you probably won’t be expected to calculate how long it takes
a radioactive nucleus to decay, but you will be expected to know how the rate of
decay works. If we take a sample of a certain radioactive element, we say that
its activity, A, is the
number of nuclei that decay per second. Obviously, in a large sample,
A will be greater than in a small
sample. However, there is a constant, called the decay constant,
?,
that holds for a given isotope regardless of the sample size. We can use the
decay constant to calculate, at a given time, t, the number of disintegrations per
second, A; the number of radioactive
nuclei, N; or the mass of the
radioactive sample, m:
A = A0e-?t
N = N0e-?t
A0,
N0,
and
m0
are the values at time t = 0. The
mathematical constant e is
approximately 2.718.
The decay constant for uranium-238 is about
5 × 10-18
s–1. After one million years, a 1.00
kg sample of uranium-238 (which has
2.5 × 1024
atoms) will contain
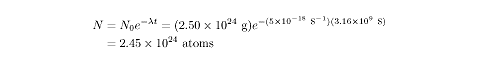
Uranium-238 is one of the slower decaying radioactive elements.
Half-Life
We generally measure the radioactivity of a certain element in terms of its
half-life,
T1/2,
the amount of time it takes for half of a given sample to decay. The equation
for half-life, which can be derived from the equations above, is:
T1/2 = In2/?
You won’t need to calculate the natural logarithm of 2—remember, no calculators
are allowed on the test. What you will need to know is that, at time
t =
T1/2,
one-half of a given radioactive sample will have decayed. At time
t = 2T1/2,
one-half of the remaining half will have decayed, leaving only one-quarter of
the original sample. You may encounter a graph that looks something like this:
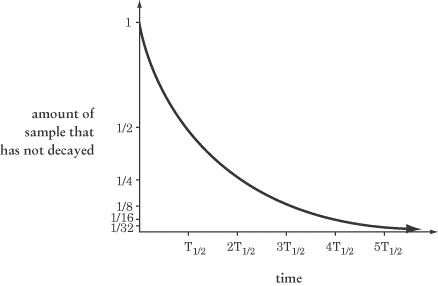
The graph of decay vs. time will get steadily closer to the x-axis, but
will never actually reach it. The fewer atoms that remain undecayed, the less
activity there will be.
Nuclear Reactions
Nuclear reactions are effectively the same thing as radioactivity: new particles
are formed out of old particles, and the binding energy released in these
transitions can be determined by the equation E
= mc2. The difference is that nuclear reactions that are
artificially induced by humans take place very rapidly and involve huge releases
of energy in a very short time. There are two kinds of nuclear reaction with
which you should be familiar for subject test Physics.
Nuclear Fission
Nuclear fission was used in the original atomic bomb, and is the kind of
reaction harnessed in nuclear power plants. To produce nuclear fission, neutrons
are made to bombard the nuclei of heavy elements—often uranium—and thus to split
the heavy nucleus in two, releasing energy in the process. In the fission
reactions used in power plants and atomic bombs, two or more neutrons are freed
from the disintegrating nucleus. The free neutrons then collide with other
atomic nuclei, starting what is called a chain reaction. By starting
fission in just one atomic nucleus, it is possible to set off a chain reaction
that will cause the fission of millions of other atomic nuclei, producing enough
energy to power, or destroy, a city.
Nuclear Fusion
Nuclear fusion is ultimately the source of all energy on Earth: fusion
reactions within the sun are the source of all the heat that reaches the Earth.
These reactions fuse two or more light elements—often hydrogen—together to form
a heavier element. As with fission, this fusion releases a tremendous amount of
energy.
Fusion reactions can only occur under intense heat. Humans have only been able
to produce a fusion reaction in the hydrogen bomb, or H-bomb, by first
detonating an atomic bomb whose fission produced heat sufficient to trigger the
fusion reaction. Scientists hope one day to produce a controllable fusion
reaction, since the abundance of hydrogen found in this planet’s water supply
would make nuclear fusion a very cheap and nonpolluting source of energy.
Back
Next
Next to display next topic in the chapter.
Practice Questions
Video Lessons and 10 Fully Explained Grand Tests
Large number of solved practice MCQ with explanations. Video Lessons and 10 Fully explained Grand/Full Tests.