Lesson: Chapter - 9
More About Entropy
For the Chemistry exam, you’ll be expected to be have an understanding of
all of the laws of thermodynamics, so to refresh your memory, the first law of
thermodynamics says that energy can neither be created nor destroyed. The
second law of thermodynamics says that the disorder of the universe, meaning
its entropy, or DS, is constantly increasing. The third law of
thermodynamics says that the entropy of a perfect crystal at 0K is zero.
What does the third law mean to you? It means that we can calculate the entropy
of any substance that’s at a temperature higher than 0K. Here are some rules
about determining the entropy of a system:
- The greater the disorder or randomness in a system, the larger the entropy.
- The entropy of a substance always increases as it changes state from solid to
liquid to gas.
- When a pure solid or liquid dissolves in a solvent, the entropy of the substance
increases.
- When a gas molecule escapes from a solvent, there is an increase in entropy.
- Entropy generally increases with increasing molecular complexity.
- Reactions that increase the number of moles of particles often increase the
entropy of the system.
Example
|
|
|
|
3.
|
Which of the following reactions results in the largest increase in
entropy? |
|
|
(A) |
CO2(s) ? CO2(g) |
|
|
(B) |
H2(g) + Cl2(g) ? 2HCl(g) |
|
|
(C) |
KNO3(s) ? KNO3(l) |
|
|
(D) |
C(diamond) ? C(graphite) |
Explanation
All of the reactions result in an increase in disorder, but A, in which
CO2 moves from a solid state to a gaseous one, represents the largest
change in disorder.
You can calculate entropy using a table of standard values in much the same way
that you calculated enthalpy earlier by using the equation below:
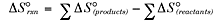
The units of entropy are J/K. The higher the S value, the more disordered
the system, so a positive (+) S value is more disordered, and a –S
value is less disordered. Remember that disorder is the favored condition,
according to the second law of thermodynamics. Now try a problem that involves
using standard S values.
Example
Calculate the entropy change at 25ºC in J/K for
2SO2(g) + O2(g) ? 2SO3(g)
given the following data:
SO2(g): 248.1 J/mol-K
O2(g): 205.3 J/mol-K
SO3(g): 256.6 J/mol-K
Explanation
[2(256.6)] - [2(248.1) + 1(205.3)] = -188.3 J/K
Don’t forget to multiply by coefficients, just as you did in enthalpy, because
the given data are still per 1 mole.
Next to display next topic in the chapter.
Practice Questions
Test Prep Lessons With Video Lessons and Explained MCQ
Large number of solved practice MCQ with explanations. Video Lessons and 10 Fully explained Grand/Full Tests.