Synthesis reaction
This is a reaction in which two or more elements or
compounds combine to form a single product. This type of reaction follows the
general equation
A + B ? C
where A and B may be either elements or compounds.
Here are some examples:
2Na(s) + Cl2(g) ? 2NaCl(s)
MgO(s) + H2O(l) ? Mg(OH)2(aq)
SO2(g) + H2O(l) ? H2SO3(aq)
Decomposition reaction
In this type of reaction, a single reactant, a
compound, breaks into two or more parts. Often these are the most difficult to
predict. Here is the general equation:
AB ? A+ B
where A and B may be either elements or compounds.
Here are some examples of decomposition reactions:
2H2O(l) ? 2H2(g)+ O2(g)
H2CO3(aq) ? H2O(l) + CO2(g)
CaCO3(s) ? CaO(s) + CO2(g)
2KClO3(s) ? 2KCl(s) + 3O2(g)
Single replacement or displacement reaction
In this type of reaction, a
more active element replaces a less active element in a compound. Among the
halogens, F2 is the most active halogen, and the activity of the
halogens decreases as you go down the group. For the metals, you will need to be
given an activity series. General equation:
A + BC ? AC + B
where A is a metal.
Here is an example of a displacement reaction in which a metal is involved:
Cu(s) + 2AgNO3(aq) ? 2Ag(s) + Cu(NO3)2(aq)
General equation:
A + BC?BA + C
where A is a nonmetal.
Here is an example of a displacement reaction where a nonmetal is involved:
Cl2(g) + 2NaI(aq)?2NaCl(aq) + I2(s)
Double replacement or displacement reaction
In this type of reaction,
two compounds react to form two new compounds. The formation of a molecular
compound such as water, the formation of a gas, or the formation of a
precipitate usually drives these reactions. Here’s the general equation:
AB + CD?AD + CB
And here are a couple of examples:
Pb(NO3)2(aq) + 2KI(aq)?2KNO3(aq)
+ PbI2(s)
HCl(aq) + NaOH(aq)?H2O(l)
+ NaCl(aq)
Combustion reaction
In this type of reaction, often a hydrocarbon is
burned in the presence of oxygen gas to form carbon dioxide (in a complete
combustion) or carbon monoxide (in an incomplete combustion, due to a limited
amount of oxygen). Here is the general equation in the presence of plenty of
oxygen:
CxHy + O2(g)?CO2(g)
+ H2O(l) or (g)
An example of this is seen when methane gas is burned in the presence of excess
oxygen (Bunsen burner reaction):
CH4(g) + 2O2(g)?CO2(g)+ 2H2O(g)
Here is the general equation for when a hydrocarbon is burned in an incomplete
combustion (oxygen is in limited supply):
CxHy + O2(g)?CO(g)
+ H2O(l)
Hydrolysis reaction
A reaction that involves water. Here is the general
equation for a hydrolysis reaction:
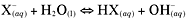
Next to display next topic in the chapter.
Practice Questions
Test Prep Lessons With Video Lessons and Explained MCQ
Large number of solved practice MCQ with explanations. Video Lessons and 10 Fully explained Grand/Full Tests.