Orbital notation is basically just another way of expressing the
electron configuration of an atom. It is very useful in determining
quantum numbers as well as electron pairing. The orbital notation for
sulfur would be represented as follows:
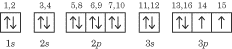
Notice that electrons 5, 6, and 7 went into their own orbitals before
electrons 8, 9, and 10 entered, forcing pairings in the 2p
sublevel; the same thing happens in the 3p level.
Now we can determine the set of quantum numbers. First, n = 3, since the valence electron (the outermost electron) is a 3p electron. Next, we know that p sublevels have an l value of 1. We know that ml can have a value between l and -l, and to get the ml quantum number, we go back to the orbital notation for the valence electron and focus on the 3p sublevel alone. It looks like this:
Simply number the blanks with a zero assigned to the center blank, with
negative numbers to the left and positive to the right of the zero.
The last electron was number 16 and “landed” in the first blank as a
down arrow, which means its ml = -1 and ms
= -1/2, since the electron is the second to be placed in the orbital
and therefore must have a negative spin.
So, when determining ml, just make a number
line underneath the sublevel, with zero in the middle, negative
numbers to the left, and positive numbers to the right. Make as many
blanks as there are orbitals for a given sublevel. For assigning ms,
the first electron placed in an orbital (the up arrow) gets the +1/2
and the second one (the down arrow) gets the -1/2.