Directions: Each set of lettered choices below refers to the numbered questions
immediately following it. Select the one lettered choice that best answers each question
and then blacken the corresponding space on the answer sheet. A choice may be used once,
more than once, or not at all in each set.
Questions 1–3
A: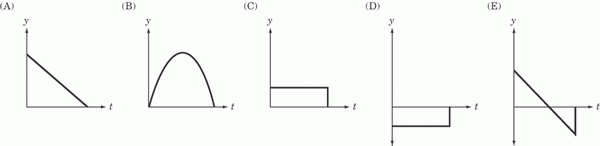
A boy throws a ball straight up in the air and then catches it again.
- Which of the above graphs best represents the ball’s position with respect to time?
- Which of the above graphs best represents the ball’s velocity with respect to time?
- Which of the above graphs best represents the ball’s acceleration with respect to time?
Explanation
You can usually answer classification questions a
bit more quickly than the standard five-choice completion
questions, since you only need to review one set of answer
choices to answer a series of questions.
The answer to question 1 is B. The ball’s position with
respect to time can be expressed by the equation y = –1/2 gt2,
where g is the downward, acceleration due to gravity. As we can see,
the graph of y against t is an upside-down parabola. In more intuitive
terms, we know that, over time, a ball thrown in the air will rise,
slow down, stop, and then descend.
The answer to question 2 is E. The acceleration due
to gravity means that the velocity of the ball will
decrease at a steady rate. On the downward half of
the ball’s trajectory, the velocity will be negative,
so E, and not A, is the correct graph.
The answer to question 3 is D. The acceleration
due to gravity is constant throughout the ball’s
trajectory, and since it is in a downward direction,
its value is negative.
Don’t worry if the question confused you
and the explanations didn’t help. This material
and more will be covered in Chapter 2: Kinematics.
This was just an exercise to show you how a classification
question is formatted.