Perhaps the easiest way to approach problems that ask you to calculate the
amounts of reactants consumed or products produced during the course of a
reaction is to start by creating a table or chart. Let’s work through a typical
example. Say the SAT II Chemistry test asks you what mass of oxygen will react
completely with 96.1 grams of propane. Notice that for this question, you’ll
need to start by writing the chemical formulas. Now follow these steps:
- Write the chemical equation.
- Calculate the molar masses and put them in parentheses above the formulas; soon
you’ll figure out you don’t have to do this for every reactant and product, just
those you’re specifically asked about.
- Balance the equation.
- Next put any amounts that you were given into the table. In this example, you
were told that the reaction started with 96.1 g of propane.
- Find the number of moles of any compounds for which you were given masses. Here
you’d start with propane: you divide 96.1 grams by the molar mass of propane
(44.11 g/mol) to get the number of moles of propane (2.18 mol).
- Use the mole:mole ratio expressed in the coefficients of each of the compounds
to find moles of all of the necessary compounds involved. The only one you
really need to know is oxygen, but let’s run through all of them for practice.
If the coefficient for propane, which is 1, is equal to 2.18 moles of propane,
then the number of moles of oxygen must be 5 × 2.18
= 10.9, the moles of CO2 is 3× 2.18
= 6.54, and the moles of H2O = 4× 2.18
= 8.72.
- Reread the problem to determine which amount was asked for. The question asks
for the mass of oxygen, so convert moles of oxygen to grams and you have the
answer:
10.9 mol × 44.01 g/mol = 349 g oxygen
|
Molar mass |
(44.11)
|
(32.00)
|
(44.01) |
(18.02) |
|
Balanced equation |
C3H8 +
|
5O2 ?
|
3CO2 + |
4H2O
|
|
Mole:mole |
1 |
5 |
3 |
4 |
|
No. of moles |
2.18 |
10.9
|
6.54 |
8.72 |
|
Amount
|
96.1 g |
349 g |
|
|
But what if this question had asked you to determine the liters of CO2
consumed in this reaction at STP (273K, 1 atm)? You would take the number of
moles of CO2 that we calculated from the table and use the standard
molar volume for a gas, or 22.4 L/mol. So, 6.54 mol × 22.4
L/mol = 146 L.
Finally, what if the question had asked how many water molecules are produced?
You would take the number of moles of water and multiply it by Avogadro’s
number, 6.02 × 1023,
to get 5.25 × 1024
molecules of water.
|
Molar mass |
(44.11)
|
(32.00)
|
(44.01) |
(18.02) |
|
Balanced equation |
C3H8 +
|
5O2 ?
|
3CO2 + |
4H2O
|
|
Mole:mole |
1 |
5 |
3 |
4 |
|
No. of moles |
2.18 |
10.9
|
6.54 |
8.72 |
|
Amount
|
96.1 g |
349 g |
146 L |
5.25 × 1024 |
You certainly don’t have to write out several tables; we just did that to make
the method clearer to you. Once you practice, you won’t need to write the
categories to the left, either. They will become second nature. In essence, you
can work the problems faster and set yourself up nicely for a clear
understanding of equilibrium problems in your future.
Perhaps your teacher at school taught you to solve this type of problem using
dimensional analysis. If so, and if you feel comfortable solving problems using
that method, don’t learn to do it our way: stick to the method with which you
feel comfortable. We’ll go through this problem using dimensional analysis:
What mass of oxygen will react with 96.1 grams of propane? Again, you would
first write the chemical formula and make sure your equation is correctly
balanced:
C
3H
8 + 5O
2 ? 3CO
2 + 4H
2O
The amount to start with, when setting up the dimensional analysis, is 96.1 g,
and your goal is to calculate the number of grams of oxygen produced:
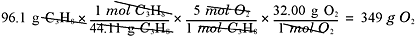
Not too hard, right? Now, how many liters of CO2 would be produced at STP?
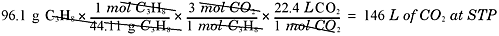
And how many water molecules are produced?
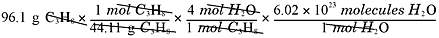

Some people prefer the table method, while others are more comfortable with
dimensional analysis. Use whatever method you feel more comfortable using, but
just be consistent—if you always do the same types of problems the same way,
you’ll feel much more confident on test day. Now try the method you prefer on
some problems.
Examples
- Solid lithium hydroxide is used in space vehicles to remove exhaled carbon
dioxide from the air; it reacts with carbon dioxide to form solid lithium
carbonate and liquid water. What mass of gaseous carbon dioxide will be consumed
in a reaction with 1.00 kg of lithium hydroxide?
Explanation
First write the reaction, then create and fill in your chart, and when you’re
done filling in the necessary blocks, it should look like this:
|
Molar mass |
(23.95)
|
(44.01) |
|
|
|
Balanced equation |
2LiOH + |
CO2 ?
|
Li2CO3 + |
H2O
|
|
No. of moles |
1000 g/23.95 g/mol = 41.8 moles |
20.9 |
20.9 |
20.9 |
|
Amount
|
1.00 kg |
20.9 × 44.01
= 920 g |
|
|
- Baking soda (NaHCO3) is often used as an antacid. It neutralizes
excess hydrochloric acid secreted by the stomach in the reaction below:
NaHCO
3(s) + HCl
(aq) ? NaCl
(aq)+ H
2O
(l?) + CO
2(aq)
How many grams of NaHCO3 would be
needed to completely react with 10.0 g of HCl?
Explanation
According to the balanced equation above, 1 mole of NaHCO3 reacts
with 1 mole of HCl. First you need to calculate how many moles of HCl are in 10
grams of HCl. The formula weight of HCl is about 36 g/mol, so you set up the
equation:

= 0.27 moles HCl.
Now, knowing that 1 mole of baking soda reacts with 1 mole of NaCl, we next need
to figure out how many grams of baking soda would react with 0.27 moles of
baking soda: 0.27 moles × 134
g/mol baking soda = 37.2 grams/mole NaHCO3
|
Molar mass |
(85.32) |
(36.46) |
|
|
|
|
Balanced equation |
NaHCO3 + |
HCl ?
|
NaCl + |
H2O + |
CO2 |
|
No. of moles |
0.274 |
10.0/36.46 = 0.274 |
|
|
|
|
Amount
|
0.274 × 85.32
= 23.4 g |
10.0 g |
|
|
|
Next to display next topic in the chapter.
Practice Questions
Test Prep Lessons With Video Lessons and Explained MCQ
Large number of solved practice MCQ with explanations. Video Lessons and 10 Fully explained Grand/Full Tests.