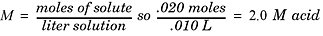A
titration (also called volumetric analysis) is a laboratory procedure
that usually involves either an acid and base neutralization reaction or a redox
reaction. In a titration, two reagents are mixed, one with a known concentration
and known volume (or a solid with a known mass) and one with an unknown
concentration. The purpose of a titration is to find the concentration of the
unknown solution. There must be some way to indicate when the two reagents have
reacted essentially completely, and at the end of the titration the unknown
solution’s concentration can be calculated since the volume of the solution
required to complete the reaction has been accurately measured.
The titrant is the solution of known concentration and is usually placed
in the burette. The burette must be rinsed with the solution to be placed in it
before filling.

The solution from the burette is added to a flask that contains either a
measured volume of a solution or a weighed quantity of solid that has been
dissolved. An indicator that changes color at or near the equivalence point is
usually added to the solution to be analyzed before titration. The solution of
known concentration is then added to the flask from the burette until the color
changes. The equivalence point is the point in the reaction where enough
titrant has been added to completely neutralize the solution being analyzed. The
end point is the point during the titration where the indicator changes
color. It is important to choose an indicator that has an end point that is at
the same pH as your expected equivalence point. The burette has graduations that
are used to read the volume of titrant that’s added to the flask.
The data required for titrations include the mass of the dry substance to be
analyzed or an accurately measured volume of the substance to be
analyzed, the initial volume and final volume of titrant required
to reach the end point, and the molarity of the titrant. At the equivalence
point, the moles of the titrant will be equal to the moles of the substance
analyzed. To obtain the moles of the unknown substance, multiply the molarity of
the titrant by the volume (in liters) of the titrant. Once moles are known, just
divide moles by volume and you have the molarity of the unknown substance
M= mole of solute/liter solution
If the substance to be analyzed is a solid, you will be trying to calculate the
molecular weight of the unknown solid. Remember that molecular weight is grams
per mole. The mass in grams will be known from the beginning of the experiment,
when the solid sample was massed. You can find the moles of the unknown
substance by multiplying the molarity of the titrant by the volume (in liters)
of the titrant. Divide grams by moles to get molecular weight
.
If you are doing a titration of a strong base with a strong acid, the
equivalence point occurs at a pH of 7.00. The dilution formula can be used to
calculate the moles of acid, which will equal the moles of base at the
equivalence point:
M1V1 = M2V2
or moles of acid = moles of base. Don’t try to use this formula if either
the acid or base is weak!
Example
Data:
|
Volume of unknown acid sample:
|
10.0 mL
|
|
Initial volume of titrant (base):
|
0.0 mL
|
|
Final volume of titrant (base):
|
20.0 mL
|
|
Molarity of titrant (base) (must be given):
|
1.0 M
|
Find the molarity of the unknown acid solution.
Explanation
Don’t forget to change mL to L.
0.020 L (titrant) × 1.0
mole/liter (titrant molarity) = 0.020 mole of base titrant
At the equivalence point: moles acid = moles base = 0.020 mole of acid