Oxidation-Reduction
Oxidation-reduction reactions involve the transfer of electrons between
substances. They take place simultaneously, which makes sense because if one
substance loses electrons, another must gain them. Many of the reactions we’ve
encountered thus far fall into this category. For example, all
single-replacement reactions are redox reactions. Before we go on, let’s review
some important terms you’ll need to be familiar with.
Electrochemistry: The study of the interchange of chemical and electrical
energy.
Oxidation: The loss of electrons. Since electrons are negative, this will
appear as an increase in the charge (e.g., Zn loses two electrons; its charge
goes from 0 to +2). Metals are oxidized.
Oxidizing agent (OA): The species that is reduced and thus causes
oxidation.
Reduction: The gain of electrons. When an element gains electrons, the charge
on the element appears to decrease, so we say it has a reduction of charge (e.g.,
Cl gains one electron and goes from an oxidation number of 0 to -1). Nonmetals are
reduced.
Reducing agent (RA): The species that is oxidized and thus causes reduction.
Oxidation number: The assigned charge on an atom. You’ve been using these
numbers to balance formulas.
Half-reaction: An equation that shows either oxidation or reduction alone.
Rules for Assigning Oxidation States
A reaction is considered a redox reaction if the oxidation numbers of the elements
in the reaction change in the course of the reaction. We can determine which elements
undergo a change in oxidation state by keeping track of the oxidation numbers as
the reaction progresses. You can use the following rules to assign oxidation states
to the components of oxidation-reduction reactions:
- The oxidation state of an element is zero, including all elemental forms
of the elements (e.g., N2, P4, S8, O3).
- The oxidation state of a monatomic ion is the same as its charge.
- In compounds, fluorine is always assigned an oxidation state of -1.
- Oxygen is usually assigned an oxidation state of -2 in its covalent compounds. Exceptions
to this rule include peroxides (compounds containing the O 22-
group), where each oxygen is assigned an oxidation state of -1, as in hydrogen peroxide
(H2O2).
- Hydrogen is assigned an oxidation state of +1. Metal hydrides are an exception:
in metal hydrides, H has an oxidation state of -1.
- The sum of the oxidation states must be zero for an electrically neutral compound.
- For a polyatomic ion, the sum of the oxidation states must equal the charge of the
ion.
Now try applying these rules to a problem.
Example
Assign oxidation numbers to each element in the following:
- H2S
- MgF2
- PO 43-
Explanation
- The sum of the oxidation numbers in this compound must be zero since the compound
has no net charge. H has an oxidation state of +1, and since there are two H atoms,
+1 times 2 atoms = +2 total charge on H. The sulfur S must have a charge of -2 since
there is only one atom of sulfur, and +2 - 2 = 0, which equals no charge.
- F is assigned an oxidation state of -1 (according to rule 3), and there are two
atoms of F, so this gives F a total charge of -2. Mg must have a +2 oxidation state
since +2 - 2 = 0 and the compound is electrically neutral.
- This time the net charge is equal to -3 (the charge of the polyatomic ion—according
to rule 7). Oxygen is assigned a -2 oxidation state (rule 4). Multiply the oxidation
number by its subscript: -2 ×4 = -8. Since there is only 1 phosphorus, just use
those algebra skills: P + -8 = -3. Phosphorus must have a +5 charge.
Example
When powdered zinc metal is mixed with iodine crystals and a drop of water is added,
the resulting reaction produces a great deal of energy. The mixture bursts into
flames, and a purple smoke made up of I2 vapor is produced from the excess
iodine. The equation for the reaction is
Zn
(s) + I
2(s) ? ZnI
2(s) + energy
Identify the elements that are oxidized and reduced, and determine the oxidizing
and reducing agents.
Explanation
- Assign oxidation numbers to each species. Zn and I2 are both assigned
values of 0 (rule 1). For zinc iodide, I has an oxidation number of -1 (group 7A—most
common charge), which means that for zinc, the oxidation number is +2.
- Evaluate the changes that are taking place. Zn goes from 0 to +2 (electrons are
lost and Zn is oxidized). The half-reaction would look like this:
Zn
0 ? Zn
2+ + 2e
-
And I2 goes from 0 to -1 (it gains electrons and so is reduced). This
half-reaction would look like this:

- Here, zinc metal is the reducing agent—it causes the reduction to take place by
donating electrons—while iodine solid is the oxidizing agent; iodine solid accepts
electrons.
Voltaic (or Galvanic) Cells
Redox reactions release energy, and this energy can be used to do work if the reactions
take place in a voltaic cell. In a voltaic cell (sometimes called a galvanic
cell), the transfer of electrons occurs through an external pathway instead of directly
between the two elements. The figure below shows a typical voltaic cell (this one
contains the redox reaction between zinc and copper):

As you can see, the anode is the electrode at which oxidation occurs; you
can remember this if you remember the phrase “an ox”—“oxidation
occurs at the anode.” Reduction takes place at the cathode, and you
can remember this with the phrase “red cat”—“reduction occurs
at the cathode.” An important component of the voltaic cell is the salt bridge,
which is a device used to maintain electrical neutrality; it may be filled with
agar, which contains a neutral salt, or be replaced with a porous cup. Remember
that electron flow always occurs from anode to cathode, through the wire that connects
the two half-cells, and a voltmeter is used to measure the cell potential in volts.
Batteries are cells that are connected in series; the potentials add to give
a total voltage. One common example is the lead storage battery (car battery), which
has a Pb anode, a PbO
2 cathode, and H
2SO
4 electrolyte
is their salt bridge.
Standard Reduction Potentials
The potential of a voltaic cell as a whole will depend on the half-cells that are
involved. Each half-cell has a known potential, called its standard reduction potential
(Eº). The cell potential is a measure of the difference
between the two electrode potentials, and the potential at each electrode is calculated
as the potential for reduction at the electrode. That’s why they’re standard
reduction potentials, not standard oxidation potentials. Here is the chart:
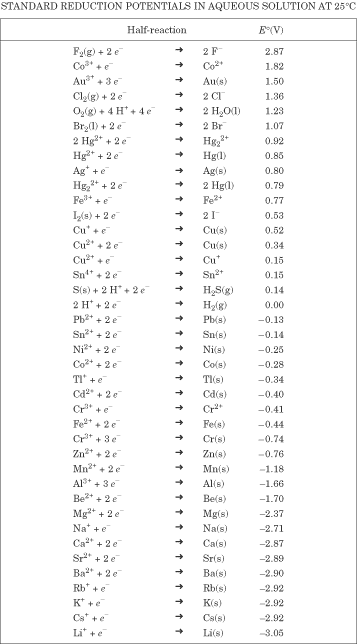
On this reduction potential chart, the elements that have the most positive
reduction potentials are easily reduced and would be good oxidizing agents (in general,
the nonmetals), while the elements that have the least positive reduction potentials
are easily oxidized and would be good reducing agents (in general, metals). Let’s
try a quick problem.
Example
Which of the following elements would be most easily oxidized: Ca, Cu, Fe, Li, or
Au?
Explanation
Use the reduction potential chart: nonmetals are at the top and are most easily
reduced. Metals are at the bottom and are most easily oxidized. Lithium is at the
bottom of the chart—it’s the most easily oxidized of all. So the order, from most
easily oxidized to least easily oxidized, is Au, Fe, Cu, Ca, Li.
Example
Which one of the following would be the best oxidizing agent: Ba, Na, Cl, F, or
Br?
Explanation
Using the reduction potential chart and the fact that oxidizing agents are the elements
that are most easily reduced, we determine fluorine is the best oxidizing agent.
Electrolytic Cells
While voltaic cells harness the energy from redox reactions, electrolytic cells
can be used to drive nonspontaneous redox reactions, which are also called electrolysis
reactions. Electrolytic cells are used to produce pure forms of an element;
for example, they’re used to separate ores, in electroplating metals (such as applying
gold to a less expensive metal), and to charge batteries (such as car batteries).
These types of cells rely on a battery or any DC source—in other words, whereas
the voltaic cell is a battery, the electrolytic cell needs a battery.
Also unlike voltaic cells, which are made up of two containers, electrolytic cells
have just one container. However, like in voltaic cells, in electrolytic cells electrons
still flow from the anode to the cathode. An electrolytic cell is shown below.

Next to display next topic in the chapter.
Practice Questions
Test Prep Lessons With Video Lessons and Explained MCQ
Large number of solved practice MCQ with explanations. Video Lessons and 10 Fully explained Grand/Full Tests.