Lesson: Chapter - 10
Organic Chemistry
Simple Organic Compounds
Organic chemistry is the branch of chemistry that studies carbon
compounds. This field is very important since carbon compounds are all around
us—they make up a wide array of common substances such as plastics, oil,
gasoline, and alcohols and are also a part of many of the foods we eat, such as
proteins, carbohydrates, and fats.
One reason that there are so many carbon compounds is because of carbon’s unique
ability for bonding. Recall the electron structure of carbon:
Carbon has four valence electrons, and these electrons can hybridize into sp3,
sp2, and sp atomic orbitals. This enables carbon to
join with other elements and be involved in single, double, and triple bonds.
Organic compounds that contain only carbon and hydrogen are called
hydrocarbons.
Some general properties of organic compounds are
- They usually have low melting points.
- They usually are nonpolar (unless they bear functional groups).
- They are usually nonconductors of electricity.
- They can exist in solid, liquid, and gaseous form. Compounds with:
- 1–4 carbons tend to be gases at room temperature; butane and propane
are among the lightest hydrocarbons and are used for fuel
- 5–10 carbons tend to be in the liquid state at room temperature; compounds that
fall in this size range are used to make gasoline and solvents
- 12–18 carbons make up jet fuels and kerosene
- More than 18 carbons tend to be solids at room temperature
Organic compounds can exist as polymers, in which many repeating units
(called monomers) make up a larger molecule. Amino acids are
monomers of proteins when amino acids are bonded in a chain, they make a
polypeptide or protein. Starches are polymers of the monomer glucose.
Plastics are polymers of organic molecules extracted from crude oil. Some common
examples include
- Polyethylene—Many ethenes strung together with covalent bonds (ethylene
is another name for ethene); shopping bags and plastic bottles are made of
polyethylene.
- Polypropylene—Many propenes strung together; glues and carpets are made
of polypropylene.
- Polystyrene—A clear, hard, brittle polymer used in CD cases; if you blow
carbon dioxide into it during manufacture and you get the soft, opaque, foamy
polymer used in a coffee cup.
Common Functional Groups
Functional groups are atoms or groups of atoms attached to an organic
compound that impart characteristic shapes and chemical properties to the
compound. There are a few functional groups that you should be able to recognize
for the SAT II Chemistry test.
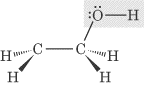
- Hydroxyl group, —OH: Compounds that contain an —OH group are considered
alcohols. An example of an alcohol, ethanol, is shown below:
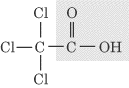
- Carboxylic acid group, —COOH: In a carboxyl group, the carbon is doubly
bonded to one oxygen and singly bonded to an OH group. An example of an organic
compound containing a carboxyl group, trichloroethanoic acid, is shown below:
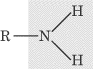
- Amine group, –NH2: An amino group contains a nitrogen
and two hydrogens. In organic chemistry, an R, like the one in the diagram
below, is often used as a shorthand notation to signify the rest of the
molecule. This notation is generally used when only a specific part of the
molecule is being discussed.
Naming Organic Compounds
Take this opportunity to look through Appendix II, Chemical Formulas Review, if
you need a refresher on how to name organic compounds. It might come in handy on
test day.
Isomerism
Isomers are compounds that have the same number and kinds of atoms but
have different structures—meaning that the atoms are arranged differently in the
molecule. Generally the number of isomers increases dramatically as the number
of carbon atoms increases because there are more options for molecular
structure.
- Different carbon skeletons (one or more bonds differ). An example is C4H10:
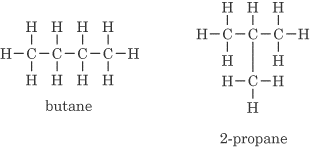
- Different functional groups. An example is C2H6O:
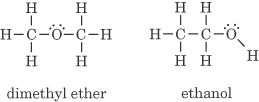
- Different positions of functional groups. An example is C3H7NH2.
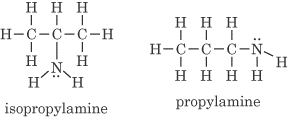
Don’t worry about memorizing all of the names of these compounds. They are only
included to show that isomers are completely different compounds, with different
names.
Simple Organic Reactions
You will be expected to be familiar with a few simple, common organic reactions
for the SAT II Chemistry exam, so we’ll go through them briefly now. Let’s start
with combustion reactions. Combustion reactions are reactions that occur
between oxygen and hydrocarbons, or CxHyOz.
There are two main types of combustion reactions—complete and incomplete.
Complete combustion occurs when excess oxygen is present; this type of
reaction produces carbon dioxide and water.
CH4 + 2O2 ? CO2+ 2H2O
Incomplete combustion occurs when a limited amount of oxygen is present,
and the products of incomplete combustion are often difficult to determine.
There may be carbon monoxide, carbon, and water or some mixture of all of these.
When cooking outdoors on a grill, you often are left with pure carbon (soot) on
utensils. Space heaters and automobiles often undergo incomplete combustion and
produce deadly carbon monoxide (CO) gas. Here’s the reaction for an incomplete
combustion:
2CH4 + 3O2 ?2CO+ 4H2O
Another common organic reaction is called an addition reaction. In an
addition reaction, two reactants join to form a single product:
H
2C = CH
2 + H
2 ?H
3C
- CH
3
Finally, we have the substitution reactions. In a substitution reaction,
one group replaces another group on the main carbon chain. The atom that’s most
commonly replaced in a substitution reaction is hydrogen. One common example of
this is halogenation, which is the addition of a halide—remember, group 7A on
the periodic table.
CH4 + Cl2 ? CH3Cl
+ HCl
Next to display next topic in the chapter.
Practice Questions
Test Prep Lessons With Video Lessons and Explained MCQ
Large number of solved practice MCQ with explanations. Video Lessons and 10 Fully explained Grand/Full Tests.